The trusted data resource for craniofacial researchers worldwide
FaceBase is a
collaborative NIDCR-funded project
.
FaceBase Data Summary
New to FaceBase?
Start here for helpful links and guided resources.
Using FaceBase
Contributing data to FaceBase
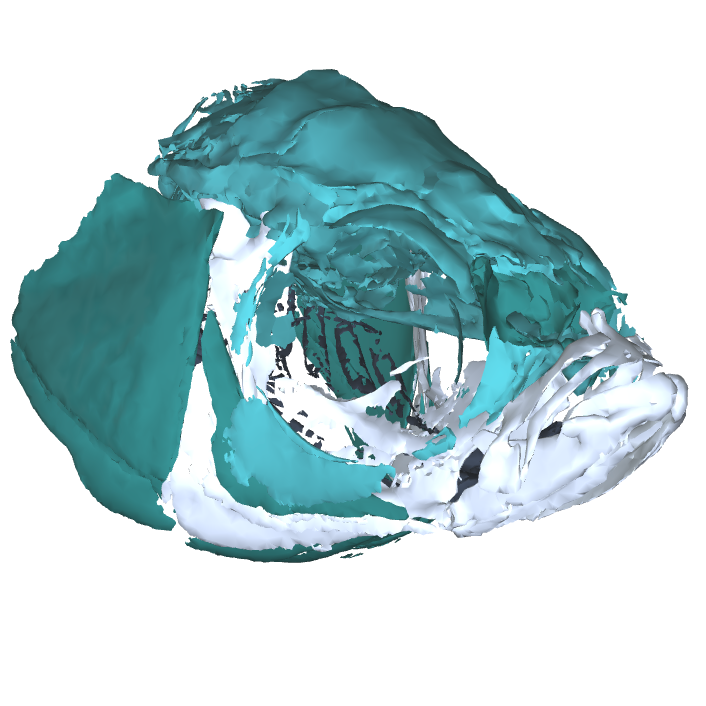
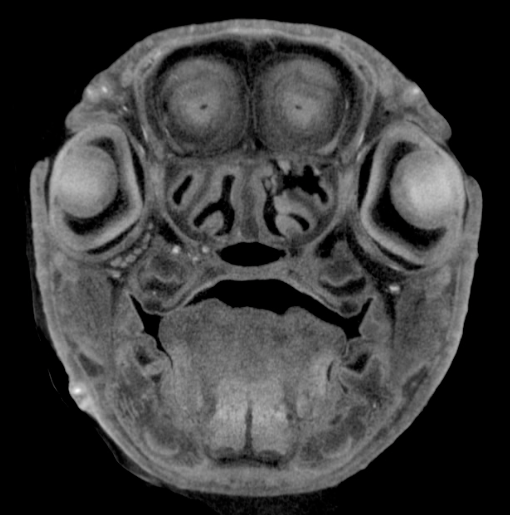
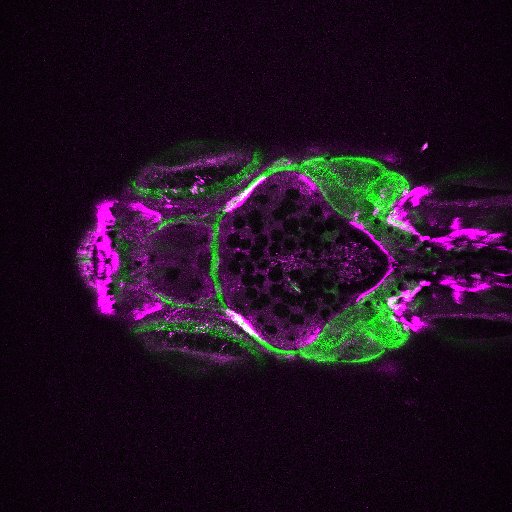
Image Search Below are some examples of the types of imaging data available in FaceBase.
News
- ALL NEWS | SIGN UP FOR OUR MAILING LIST
-
05/21/2025 -
Data release: SHH expression in the Frontonasal Ectodermal Zone
-
05/20/2025 -
Data release: TGF-β Signaling and Craniofacial Morphogenesis
-
03/26/2025 -
FaceBase Achieves CoreTrustSeal Certification
-
03/11/2025 -
Join us at AADOCR in New York City this week!
-
03/07/2025 -
Registration is open for the FaceBase 2025 Community Forum!
-
11/04/2024 -
Creating protection for children and adolescents using FaceBase data
Publications
-
Mok, Chan Hee; Hu, Diane; Losa, Marta; Risolino, Maurizio; Selleri, Licia; Marcucio, Ralph S.. PLOS Genetics. vol. 21(5), e1011315. May 2025.
Sonic hedgehog (SHH) signaling from the frontonasal ectodermal zone (FEZ) is a key regulator of craniofacial morphogenesis. Along with SHH, pre-B-cell leukemia homeobox (PBX) transcription factors regulate midfacial development. PBXs act in the epithelium during fusion of facial primordia, but their specific interactions with SHH have not been investigated. We hypothesized that PBX1/3 regulate SHH expression in the FEZ by activating or repressing transcription. The hypothesis was tested by manipulating PBX1/3 expression in chick embryos and profiling epigenomic landscapes at early developmental stages. PBX1/3 expression was perturbed in the chick face beginning at stage 10 (HH10) using RCAS viruses, and the resulting SHH expression was assessed at HH22. Overexpressing PBX1 expanded the SHH domain, while overexpressing PBX3 resulted in an opposite effect. Conversely, reducing PBX1 expression decreased SHH expression, but reducing PBX3 induced ectopic SHH expression. We performed ATAC-seq and mapped binding of PBX1 and PBX3 to DNA with ChIP-seq on the FEZ at HH22 to assess direct interactions of PBX1/3 with the SHH locus. These multi-omics approaches uncovered a 400 bp PBX1-enriched element within intron 1 of SHH (chr2:8,173,222–8,173,621). Enhancer activity of this element was demonstrated by electroporation of reporter constructs in ovo and luciferase reporter assays in vitro . When bound by PBX1, this element upregulates transcription, while it downregulates transcription when bound by PBX3. The present study identifies a cis- regulatory element, named SFE1, that interacts with PBX1/3 either directly or within a complex with cofactors to modulate SHH expression in the FEZ. This research establishes that PBX1 and PBX3 play complementary roles in SHH regulation during embryonic development. -
Feng, Jifan; Janečková, Eva; Guo, Tingwei; Ziaei, Heliya; Zhang, Mingyi; Geng, Jessica Junyan; Cha, Sa; Araujo-Villalba, Angelita; Liu, Mengmeng; Ho, Thach-Vu; Chai, Yang. Nature Communications. vol. 16(1), 4396. May 2025.
-
Bobzin, Lauren; Nickle, Audrey; Ko, Sebastian; Ince, Michaela; Huang, Aaron; Bhojwani, Arshia; Roberts, Ryan; Merrill, Amy E.. Development. vol. 152(2), dev204264. January 2025.
The calvarial bones of the infant skull are linked by transient fibrous joints known as sutures and fontanelles, which are essential for skull compression during birth and expansion during postnatal brain growth. Genetic conditions caused by pathogenic variants in FGFR2, such as Apert, Pfeiffer, and Crouzon syndromes, result in calvarial deformities due to premature suture fusion and a persistently open anterior fontanelle (AF). In this study, we investigated how Fgfr2 regulates AF closure by leveraging mouse genetics and single-cell transcriptomics. We find that AF cells, marked by the tendon/ligament factor SCX, are spatially organized into ecto- and endocranial domains that selectively differentiate into ligament, bone, and cartilage to form the posterior frontal suture. We show that AF cell differentiation is non-autonomously regulated by FGFR2 signaling in osteogenic front cells of the frontal bones, which regulate WNT signaling in neighboring AF cells by expressing the secreted WNT inhibitor Wif1. Upon loss of Fgfr2, Wif1 expression is downregulated, and AF cells fail to form the posterior frontal suture. This study identifies an FGF-WNT signaling circuit that that directs suture formation within the AF during postnatal development.